List of pharmaceutical products
Pharmaceuticals (OTC)
Class 1 medicines
(iii) Drugs designated by the Minister of Health, Labour and Welfare as those that are likely to cause damage to health to the extent that they interfere with daily life due to their side effects, etc., for which special attention is required in relation to their use, and drugs that were deemed to fall under Article 14(8)(i) of the Pharmaceutical Affairs Act when the application for approval of their manufacture and sale was filed and for which a period specified by the Ordinance of the Ministry of Health, Labour and Welfare has not passed since the approval pertaining to said application was granted. (2) Those for which a period specified by an Ordinance of the Ministry of Health, Labour and Welfare has not yet elapsed since the approval pertaining to the application was granted.


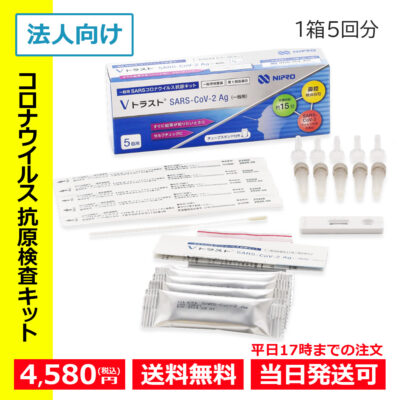
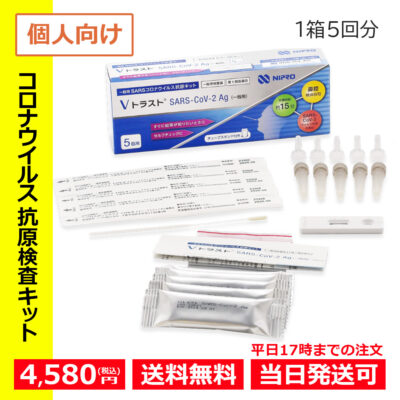

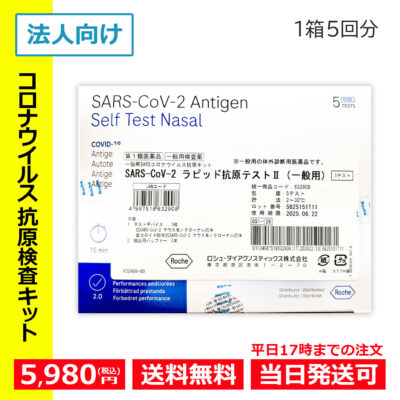
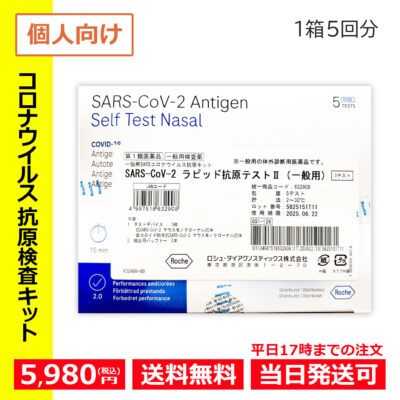


- Hot





Designated category 2 medicines
Class 2 drugs designated by the Minister of Health, Labour and Welfare as requiring special attention.












Class 2 medicines
Medicinal products (excluding class 1 medicinal products) which may cause health hazards to the extent that their side effects or other adverse effects interfere with daily life, and which are designated by the Minister of Health, Labour and Welfare.


- Hot











- Hot


- Hot

- Hot

- Hot














Class 3 medicines
OTC medicines other than class 1 and 2 medicines.











- Hot







designated quasi-drug
Items that have a mitigating effect on the human body and are not machinery or equipment.







Recommended products

- Hot

- Hot

- Hot

- Hot

- Hot

- Hot

- Hot

- Hot
